We seek to understand the basic processes that impact air quality and climate. This understanding comes from asking a series of questions surrounding climate chemistry and air pollution, as outlined below.
Air Pollution
1. How do greenhouse gas emissions and air quality vary at the neighborhood scale?
The majority of people on our planet now, or soon will, live in cities. Within city activities, including personal travel, the movement of goods, home heating, cooking, consumer products and the activities of urban industries are all major sources of emissions that contribute to growing concentrations of greenhouse gases and serve as precursors to poor air quality. Efforts to reduce such emissions are proceeding apace driven by local, regional and national policies, consumer demand for “greener” products and concerns for public health and the climate. Despite the ambitious goals set out by mayors and municipalities in reducing greenhouse gas emissions, the monitoring and understanding of actual reductions in greenhouse gases in the urban atmosphere have lacked the granularity necessary to provide useful information to citizens and policymakers.
To address these limitations in our surface and space-based observing systems, over the past few years we have established the Berkeley Environmental Air Quality and CO2 Observation Network, BEACO2N. BEACO2N is an instrument for observing and mapping the composition of the urban atmosphere with ~2km spatial resolution. It currently includes observations of CO2, CO, NO2, NO, O3, and particles at ~70 locations spaced by about 2km in the San Francisco Bay Area (Figure 2), 15 in Houston, TX, 10 in New York City and soon ~25 in Glasgow, Scotland and 12 in Los Angeles. BEACO2N is a cost effective and scalable approach to determining the total emissions of various chemicals into the urban atmosphere, their distribution in space with neighborhood and sectoral precision, and their trends over time.
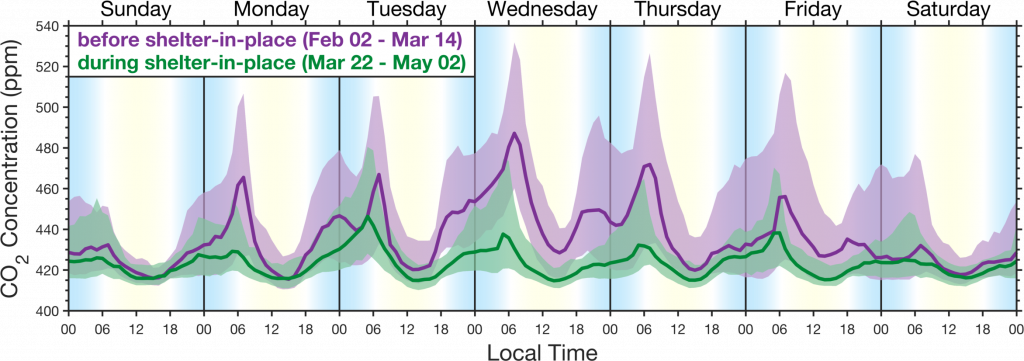
The figure above demonstrates the precision of the BEACO2N network in capturing emission changes at the neighborhood scale, resulting from the shelter in place order issued on March 16th for the San Francisco Bay Area.
Climate Chemistry:
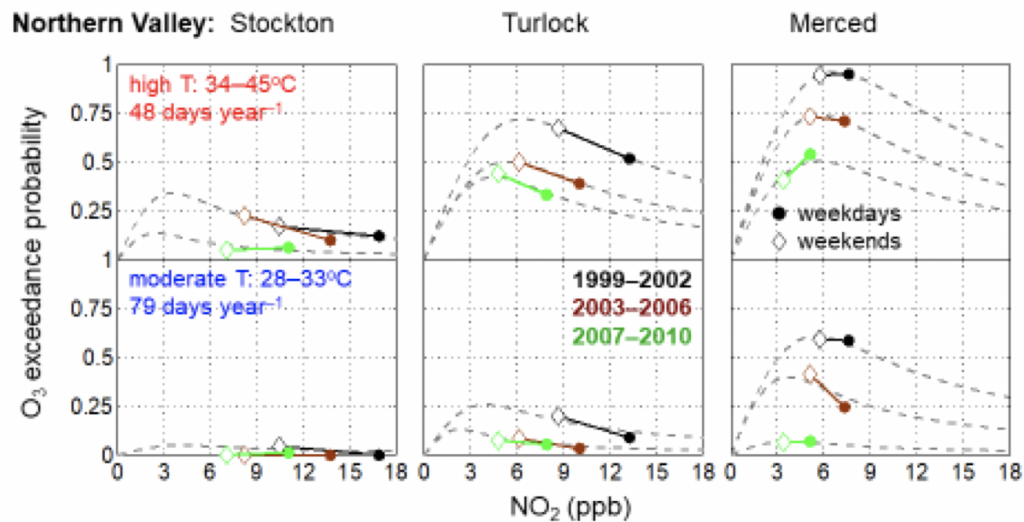
1. How do temperature extremes affect the chemistry of the atmosphere?
Using laboratory and observations in the field, we’ve identified factors that contribute to high ozone events, which are events in time where ozone levels are dangerously high. Our lab has explored how the mechanisms driving these high ozone events differ in winter and summer. This research allows us to understand and predict the frequency of high ozone events in a future, warmer climate.
2. What are the controls on the lifetime of HOx?
The reaction system between Isoprene-RO2 + HO2 is not well understood. Reactive nitrogen oxides are fundamental to the chemistry of atmospheric oxidation and affect air quality, climate, and ecosystem nutrient cycling. Nitrogen oxides interact nonlinearly with HOx radicals (HOx ≡ OH + HO2 + RO2) to both catalyze ozone (O3) formation and terminate the HOx catalytic chain and suppress O3. This interplay doubly affects the formation of secondary organic aerosol (SOA) by controlling the rate of organic oxidation and also the balance between organic peroxide and organic nitrate production, products of VOC oxidation with distinctly different propensities for incorporation into aerosol. Quantifying this chemistry is of consequence as O3 is a toxic air pollutant, a greenhouse gas, an oxidant itself, and the major source of the hydroxyl radical (OH), the primary atmospheric oxidant. Likewise, the total aerosol burden, a large fraction of which is SOA, is known to cause adverse health effects and to influence climate both directly and indirectly by altering the Earth’s radiation balance.
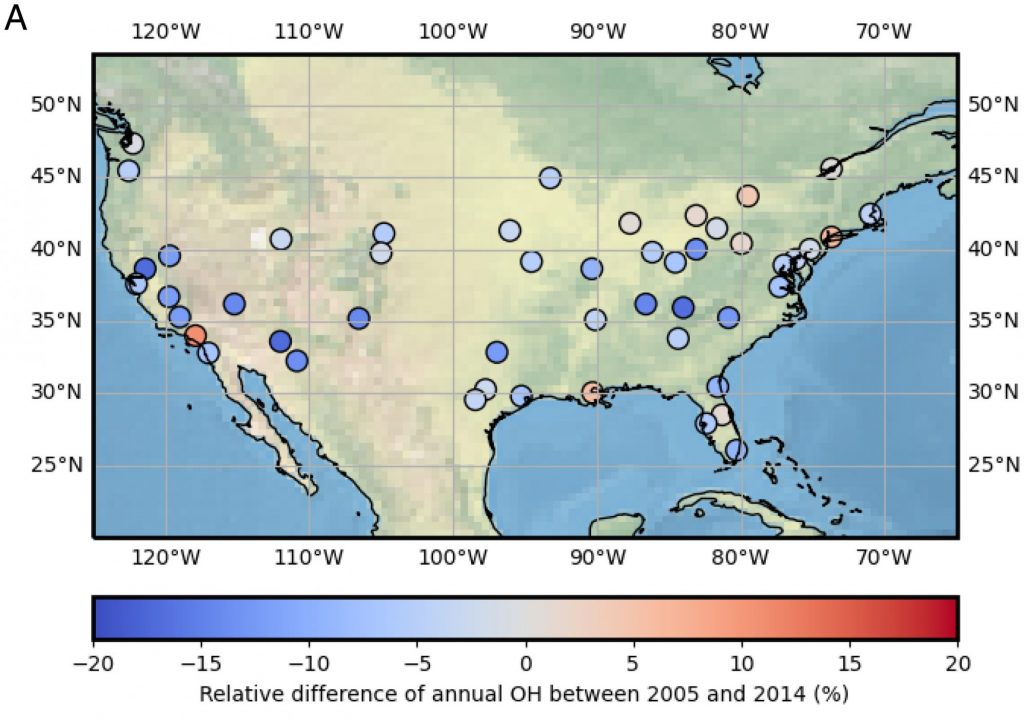
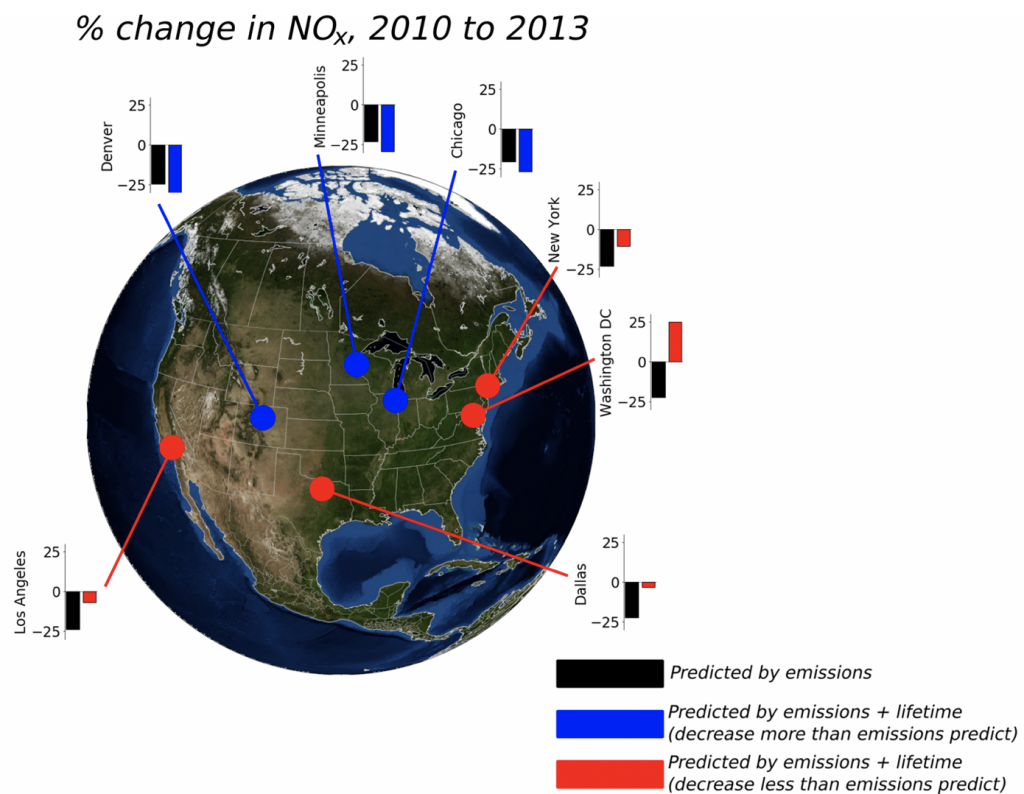
3. What are the controls on the lifetime of NOx?
NOx lifetime relates nonlinearly to its own concentration; therefore, by observing how NOx lifetime changes with changes in its concentration, inferences can be made about the dominant chemistry occurring in an urban plume. We use satellite observations of NO2 from high-resolution products to observe changes in NOx lifetime across decades of observations.
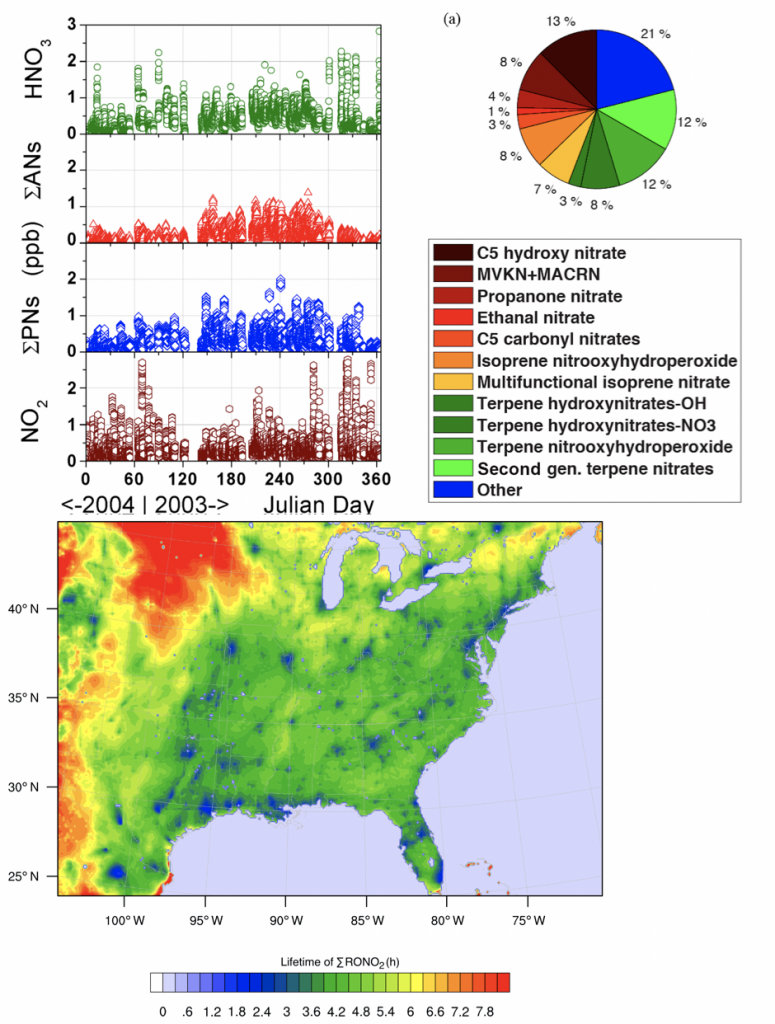
4. What is the significance of RONO2 chemistry, particularly as it relates to the lifetime of NOx?
A better understanding of the chemistry of nitrogen oxides (NONOx) is crucial to effectively reducing air pollution and predicting future air quality. The response of NOx lifetime to perturbations in emissions or in the climate system is set in large part by whether NOx loss occurs primarily by the direct formation of HNO3 or through the formation of alkyl and multifunctional nitrates (RONO2). Over the past decades, changes in anthropogenic emissions of both NONOx and VOCs have led to a significant increase in the overall importance of RONO2 chemistry to NONOx loss. We find that this shift is associated with a decreased effectiveness of NONOx emissions reductions on ozone production in polluted areas and increased transport of NONOx from source to receptor regions. This change in chemistry, combined with changes in the spatial pattern of NONOx emissions, is observed to be leading to a flatter distribution of NO2 across the United States, potentially transforming ozone air pollution from a local issue into a regional one.